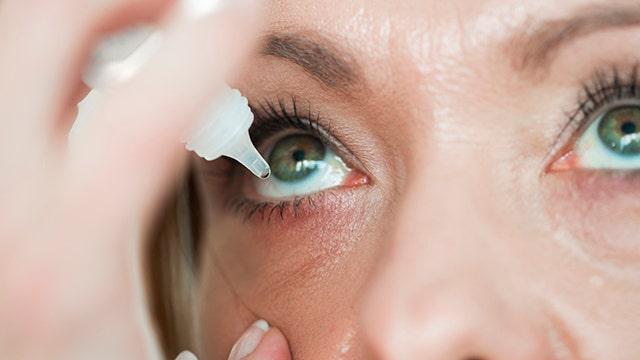

A brand of over-the-counter eye drops may be linked to a bacterial infection that left one person dead and three others with permanent vision loss, according to the Center for Disease Control and Prevention.
The CDC has identified at least 50 people in 11 states with Pseudomonas aeruginosa, which is a type of bacterium resistant to most antibiotics. So far, there have been cases in California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah and Washington.
The agency said it is investigating, and that a majority of people affected reported using preservative-free EzriCare Artificial Tears before they became infected, according to a Jan. 20 statement.
Among the reported cases, 11 people developed eye infections, including at least three who were blinded in one eye. Others who became ill had respiratory infections or urinary tract infections, and one person died after the bacterium entered their bloodstream.
It remains unclear at this time if those affected had underlying eye conditions, such as glaucoma or cataracts, that would have made them more susceptible. Eye infection symptoms include pain, swelling, discharge, redness, blurry vision, sensitivity to light and the feeling that an object is stuck in the eye.
Pseudomonas aeruginosa bacteria are commonly found in water, soil and on the hands of otherwise healthy people. These infections typically take place in hospitals among people with weakened immune systems. This type of bacterium is often resistant to standard antibiotics.
The eye drops in question are labeled as preservative-free, meaning the product does not contain anything that could prevent microbiological growth.
It is possible that the drops were contaminated during the manufacturing process or when a person with the bacteria on their skin opened the container.
The CDC discovered the bacteria in the eye drop bottles and is conducting tests to determine whether that bacteria matches the strain found in patients.
EzriCare Artificial Tears had not been recalled as of Tuesday evening.
The CDC is recommending that clinicians and patients stop using the product until the investigation and laboratory analysis are complete.
